News Story
Better, Cheaper COVID-19 Tests
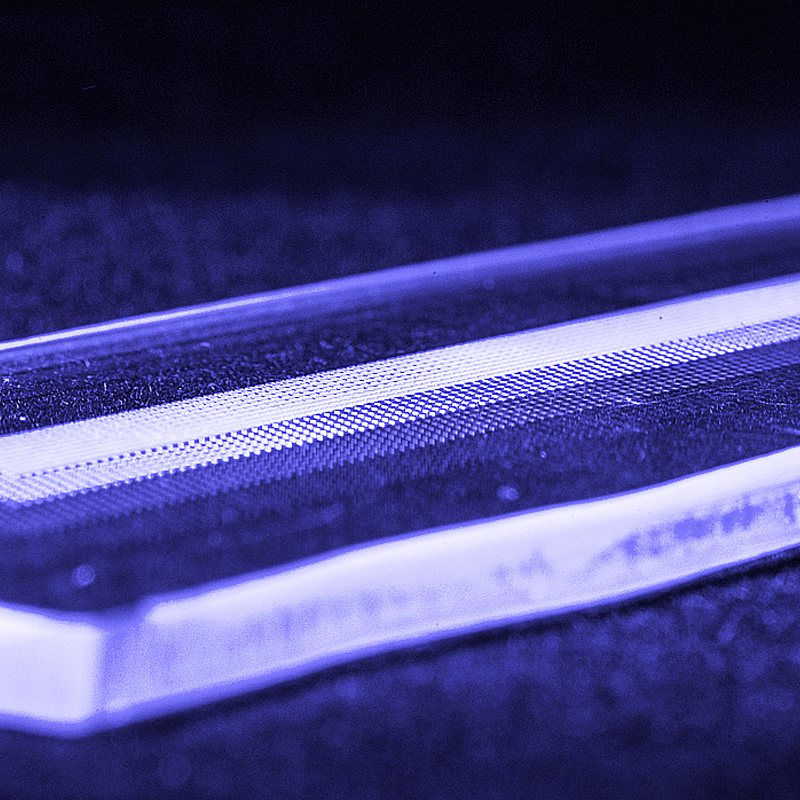
This small plastic chip could help detect multiple COVID-19 nucleic acid targets cheaply and quickly.
One of the biggest obstacles to easing COVID-19 restrictions is the lack of ready access to large-scale, inexpensive, and accurate testing to effectively and efficiently diagnose and track the virus.
To date, testing efforts have been largely inconsistent and the diagnostic tools available for COVID-19, are expensive and rely on costly and bulky readers to process.
In addition, the current gold standard test—reverse transcription polymerase chain reaction (RT-PCR)—can take hours to yield a result, and that is only if resources are available to immediately process the test. Unfortunately, doctors’ offices lack the lab resources for the test, and even in hospitals with the appropriate in-house labs, demand far outpaces capacity.
The most commonly used testing model today involves collecting samples at testing sites. At the end of the day, samples are picked up and shipped to a lab—often in another state—where they are tested as equipment becomes available. Under these conditions, patients often wait a week or more to find out if they test positive for COVID-19.
Unfortunately, time is critical. Faster test results would allow patients and their caregivers to make better-informed decisions about a course for treatment. Speed and cost are also important to support routine and broad scale testing to improve outbreak management.
Recognizing this need, Professor Don DeVoe and Associate Professor Ian White in the A. James Clark School of Engineering’s departments of mechanical engineering and bioengineering are working to develop an exceptionally cost-effective and rapid diagnostic test for SARS-CoV-2, the virus that causes COVID-19.
The team’s approach relies on a small plastic chip that incorporates microfluidics technology. With an array of microwells containing different chemical reagents, the chip could detect multiple COVID-19 nucleic acid targets. The technology leverages a National Institutes of Health (NIH) research project (R01) grant that DeVoe and White are using to develop a novel technique for automating the integration of reagents and isolation of samples into multiple microwells to make a rapid testing system possible.
To test for COVID-19 and other respiratory diseases, the team’s system utilizes a compact and reusable reader. This proprietary reader takes the place of existing test readers that cost in excess of $10,000 and are bulky, the size of a tabletop printer or larger.
The team’s compact reader and disposable chips could provide reliable test results in less than 15 minutes.
“The microfluidic technology is highly scalable, allowing us to increase the number of assay targets without any extra work,” explained DeVoe. “This will not only allow us to quantify multiple gene target for SARS-CoV-2 in a single step, but in the future we can implement a full respiratory panel in one test.”
“The microfluidic technology is highly scalable, allowing us to increase the number of assay targets without any extra work. This will not only allow us to quantify multiple gene target for SARS-CoV-2 in a single step, but in the future we can implement a full respiratory panel in one test."
Professor Don DeVoe
The ability to test for multiple viral pathogens is another improvement over current testing models, and it could help medical workers pivot their treatment approach based on real-time data.
The team estimates that their testing system—chip, biological materials, and readers—would cost less than $8 per test. At this price, the team’s hope is that the system would provide an accurate, compact, and inexpensive means of testing that could be widely deployed in urgent care facilities—as well as at drive-thru testing sites—where resources and space is limited.
“The key to application in the field like this is not only the low per-test cost, but also, the ease of use,” explains White. “While many microfluidic designs have failed to achieve instrument-free operation or to eliminate precise manual steps, the microfluidic approach satisfies the key user needs and regulatory requirements.”
DeVoe also points out that, because the test is low-cost and easy-to-use, it could prove valuable in tracking community spread by allowing individuals within the general population to be tested regularly, regardless of whether or not they exhibit symptoms.
Beyond that, the team envisions that the test could even be available at the consumer level, enabling individuals to self-test at home.
Before any of these applications can go into practice, the team has a few more challenges to address. The chip’s microfluidics technology—developed by chemical and biomolecular engineering Ph.D. student Supriya Padmanabhan—must be optimized for use with the chemical reagents required for efficient identification of COVID-19.
Meanwhile, along with DeVoe, three undergraduate researchers, Raymond Koehler (ME), Mani Pabba (CEE) and Gamitha Wijekoon (ME), are validating components for their compact reader.
The team’s test may not be ready for commercialization for a year or more, but their system could prove useful in a world where the “new normal” requires wide-scale, economical testing of COVID-19.
“To get our country back to a high level of functionality, improved surveillance will be critical to knowing who is exhibiting an active infection, symptomatically or not,” adds DeVoe. “And since we don’t yet know if or when a vaccine can be realized, or even if we will develop herd immunity through prior infection, it is critical that we have a way to test a lot of people cheaply and frequently.”
For more information about this project and other COVID-19-related research put forth by the A. James Clark School of Engineering, please visit clark.covid.umd.edu.
Published May 19, 2020