News Story
CelestRay Secures FDA Clearance for Implantable Medical Device with Help from Fischell Institute

The Robert E. Fischell Institute for Biomedical Devices has successfully guided Maryland startup CelestRay Biotech Company, LLC in acquiring FDA 510(k) clearance for its synthetic membrane implant, which aids in the healing of tendon repairs.
The clearance, which paves the way for CelestRay to legally market its implant, also marks the first medical device aided to market by the Fischell Institute since its launch in 2019.
“Our mission is to transform biomedical device research into real commercial products,” said Fischell Institute Director William Bentley. “We are pleased to hit this milestone, and it is our hope that CelestRay’s device can help persons with ruptured tendons heal in an effective manner.”
Called SpinMedix, CelestRay’s device is an implantable synthetic membrane, made of biodegradable polymers, that wraps around a ruptured tendon during its repair. The membrane helps prevent the scarring of a tendon that could impede its ability to slide naturally.
Fischell Institute Research Professor Lex Schultheis guided CelestRay throughout the clearance process, identifying the studies to be performed, the scientific protocols, and the analysis of the data that the FDA reviewed.
“CelestRay got a CRO [contract research organization] to do the experiments, we went over the protocols, and they made sure they adhered to industry standards,” said Schultheis. “All testing was done according to Good Laboratory Practice (GLP) protocols. Overall, we were pleased to be a trusted partner for advice and consulting on this project.”
Based in Bethesda, Md. CelestRay was co-founded by Charles Han and Shanshan Xu. Han worked at NIST for three decades, serving first as a research scientist and later as the leader of the polymer blends and multiphase materials groups. He was elected a NIST Fellow in 1995. Han is currently a Visiting Research Scientist in the Clark School’s Department of Materials Science and Engineering, while Xu is a Visiting Associate Research Professor.
Han connected with the Fischell Institute through Robert Briber, Special Assistant to the President for Strategic Initiatives and Associate Dean for Research, A. James Clark School of Engineering, who worked with Han at NIST. Briber provided polymer expertise to CelestRay.
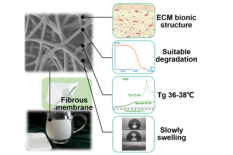 “The company’s membrane is formed through electrospinning,” said Briber. “CelestRay can control both the porosity and thickness of the material, and at a certain temperature, it spontaneously curls up and wraps the tendon to be repaired. Surgeons will not have to roll it themselves or glue it.”
“The company’s membrane is formed through electrospinning,” said Briber. “CelestRay can control both the porosity and thickness of the material, and at a certain temperature, it spontaneously curls up and wraps the tendon to be repaired. Surgeons will not have to roll it themselves or glue it.”
SpinMedix is CelestRay’s first product.
"This achievement is a testament to our pursuit of innovation and excellence in the field of bioengineering,” said Xu. “The 510(k) clearance validates our efforts and opens new doors for us to significantly impact patient care for a broader medical community.”
That community could expand further as the company plans to grow.
"We feel both humbled and invigorated by this recognition, and we look forward to continuing our work with the Fischell Institute to explore new biomedical device engineering frontiers," Han and Xu said jointly.
CelestRay’s SpinMedix implant was developed in China.
The Fischell Institute is a unit of the A. James Clark School of Engineering at the University of Maryland.
Published September 21, 2023