News Story
UMD Research Team Makes Strides in Immunotherapy of Cancer
University of Maryland bioengineers have established an approach that could treat large tumors in a targeted way that decreases the need for chemotherapy and other highly invasive approaches like surgery. Their results, published in late January in the journal Nature Communications, represent a new landmark in their efforts to design an immunotherapy strategy to treat advanced cancer.
The researchers performed their studies on breast cancer, the most commonly diagnosed cancer worldwide. The disease accounts for 1 in 8 cancer diagnoses worldwide. Globally in 2020, there were about 2.3 million new cases of breast cancer and about 685,000 deaths from the disease.
Current clinical treatments for breast tumors include cryosurgery (also called cryotherapy and cryoablation), the method in the team’s research. Minimally invasive, with negligible cosmetic damage, cryotherapy is done in an outpatient setting. But the technique, which uses extreme cold to “frostbite” cancer cells and abnormal tissue, can only treat tumors around two centimeters in size or smaller. In larger tumors, cryosurgery cannot currently tackle metastatic tumors.
Across nearly all types of cancer, the number one cause of death is not the primary tumor itself, rather, it is the spread of cancer to other parts of the body. This spread, known as metastasis, occurs when cancer cells break away from a primary tumor site and travel via the bloodstream or the lymphatic system to form new tumors in other parts of the body.
Instead of chemotherapy, surgery, or other invasive approaches to treatment, study first author and Fischell Department of Engineering (BIOE) postdoc Wenquan Ou, Professor Xiaoming (Shawn) He, and other members of He’s Multiscale Biomaterials Engineering (MBE) Laboratory are exploring ways to use cryotherapy to efficiently and safely treat more advanced cancer. The University of Maryland scientists hope that with their new approach, outpatient cryosurgery will be possible for not only localized but also metastatic tumors.
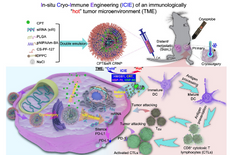
“What kills breast cancer patients is advanced cancer with metastasis. But, advanced breast cancer patients can’t benefit from the many advantages of the minimally invasive cryosurgery yet,” said He. “Naturally, the immune system kills mutated cells to prevent cancer throughout the human body. However, some mutated cells may evade the immune system, leading to cancer. Cryosurgery has been shown to induce cryo-immune response that kills cancer cells in the primary tumor with cryosurgery, but it is insufficient to kill metastatic cancer cells away from the site of cryosurgery. This drives us to develop novel strategies to potentiate the cryo-immune response for destroying both primary and metastatic cancer cells.”
Cancer immunotherapy is already a promising strategy for cancer treatment, functioning by rallying the immune system to recognize and attack tumors. But the immunosuppressive (known as immunologically “cold”) microenvironment within tumors restricts the immune system’s actions.
To explore this situation, the team looked to RNA interference (RNAi) with small interfering RNA (siRNA), a biological process by which RNA molecules inhibit gene expression or translation. This process can be used to precisely target virtually any genes — including those that may contribute to cancer growth. However, siRNAs are extremely unstable; enzymes that degrade them are highly present in the human body.
To address this challenge, the team developed an innovative cold-responsive nanotechnology that not only can protect siRNAs in human body, but also deliver them into cancer cells. That’s where siRNAs perform their function in response to cryosurgery’s cold temperature. At the same time, the scientists used innovative nanotechnology to deliver a chemotherapy drug.
The results are groundbreaking: The research team observed that the ratio of cancer-cell-killing to immunosuppressive T cells increases by more than 100 times in primary tumors experiencing cryosurgery, as well as in tumors away from the cryosurgery site. The treatment leads to cell death and further activates more T cells to kill metastatic breast cancers.
For this work, He and members of his lab teamed up with researchers from the University of Maryland’s Marlene and Stewart Greenebaum Comprehensive Cancer Center, Indiana University School of Medicine, and the National Cancer Institute to shed light on treatment of larger tumors with cold technology.
Funded in part by a grant from the National Institutes of Health, He and his team found inspiration in previous cold technology work on smaller tumors and how our bodies typically respond to infection.
“It is interesting that by leveraging cold-responsive nanotechnology, the physical cold (i.e., our freezing techniques) can turn immunological cold (immunosuppressive) into immunological hot (immuno-active). This is an exciting finding—potentially making cryosurgery much more precise, with broader applications,” said He. “It’s very encouraging that now we can potentially treat not just a localized tumor but a metastatic tumor with cryosurgery.”
In addition to Ou and He, National Science Foundation (NSF) graduate fellow Samantha Stewart and 10 other researchers contributed to the paper.
Written by Catherine Arnold
Published February 22, 2023









